Charsire New Drug BAC for
DEMENTIA
The responder groups of Charsire’s Alzheimer’s Disease and Vascular Dementia new drug BAC has been identified in the clinical trial. The responder trend started at week 4 and stabilized through week 12.
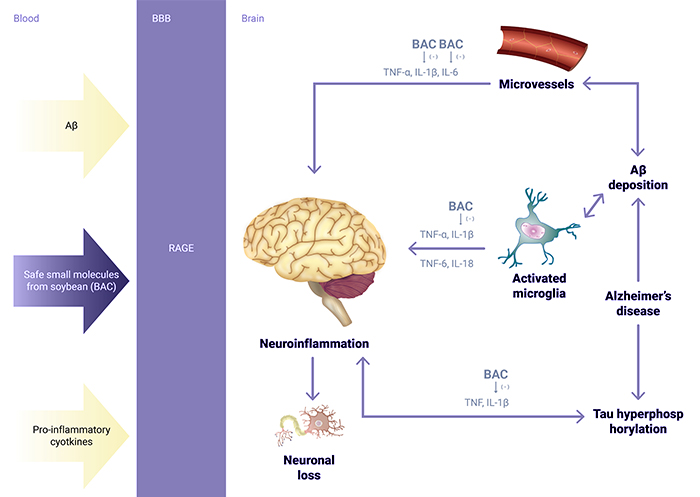
Recent pivotal clinical trials of drug candidates that target amyloid beta and Tau protein have failed as a result of not knowing the exactly mechanisms that give rise to the pathology of Alzheimer’s Disease. The pharmacological studies of BAC indicated that BAC may improve Alzheimer’s Disease and Vascular Dementia conditions through anti-inflammatory routes.

Improvement in effective group

Toxicology tests for phase II, completed

Phase II clinical study approved by US FDA, completed
Overview & Medication Predicament::
Challenges ahead for new drug developments, current medications provide only palliative treatment
Ambiguous mechanism of dementia pathology is the main reason why new drug development faced onerous challenges and resulted in efficacy of current medications being functionally defined as palliative, as oppose to cure. In general, dementia patients when treated with currently available medications, receive a moderate efficacy while bear strong side effects. These repeated problems cause huge burden to the families and caregivers of patients and the society overall.
Results of recent pivotal clinical trials have suggested that focusing only on amyloid beta and Tau protein may faltered. The market is in desparate need of a new drug with high quality treatment outcomes and significantly less side effects to offer solutions to reduce dementia issued faced by all affected mankind.
Core Technology of BAC
The pharmacological studies indicated that BAC may improve dementia conditions through a mechanism of action of reducing pro-cytokines to minimize inflammation in the brain which is different from the more commonly hypothesized mechanism of action of both amyloid beta and Tau protein.
Dementia needs more than care
Fourty seven million people worldwide are currently living with dementia, and there are 10 million new cases each year. In 2018, more than 16 million family members and other unpaid caregivers provided an estimated 18.5 billion hours of care to people with Alzheimer’s or other dementias. This care is valued at nearly $234 billion, which is equivalent to the 18th largest economy by GDP in the world.
BAC Applications
Other than Alzheimer‘s Disease and Vascular Dementia, Charsire’s BAC can be applied to other brain damage and stroke associated problems, such as Parkinson’s disease, epilepsy, facial paralysis, sleep disorders, and emotional problems.
